Passive and active implantable devices are subjected to high rates of severe fibrotic reaction.
No effective strategy is clinically available to prevent this fibrotic reaction.
This fibrotic reaction, if untreated, presents significantly negative consequences for the patient.
MYcoat™ addresses a medical problem. Once an implant is inserted into the body, the body's immune response calls for fybroblasts near the implant to activate, turning into myofibroblasts. These myofibroblasts, in turn, attach to the implant in a process known as "docking". Over time, this "docking" causes deterioration of the implant's function due to capsular contraction.

We developed MYcoat™ to specifically address this issue. MYcoat™ mimicks human tissue and sets up the architecture around the implant in order to effectively prevent the activation of fibroblasts, thus significantly reducing and, in some cases, preventing "docking". This, in turn, helps to prevent implant capsular contraction, helping the patient to reduce exposure to experiencing pain, infections, increased medical costs and loss of function of the implant device.
We developed MYcoat™ to humanize implants.
During the first experiments In-Vitro, the labseed team demonstrated:
More details in:
Majd, H., et al., Biomaterials 54 (2015), pp. 136-147
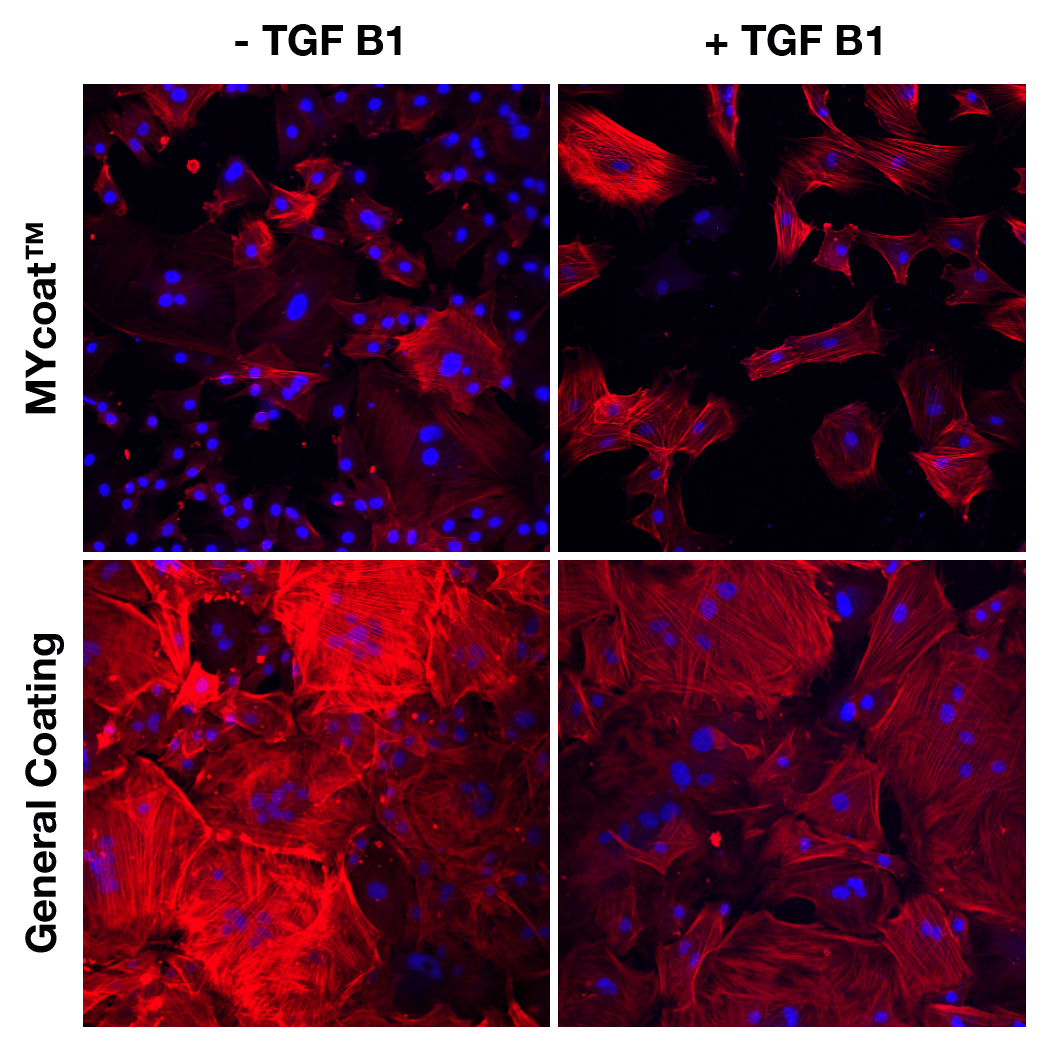
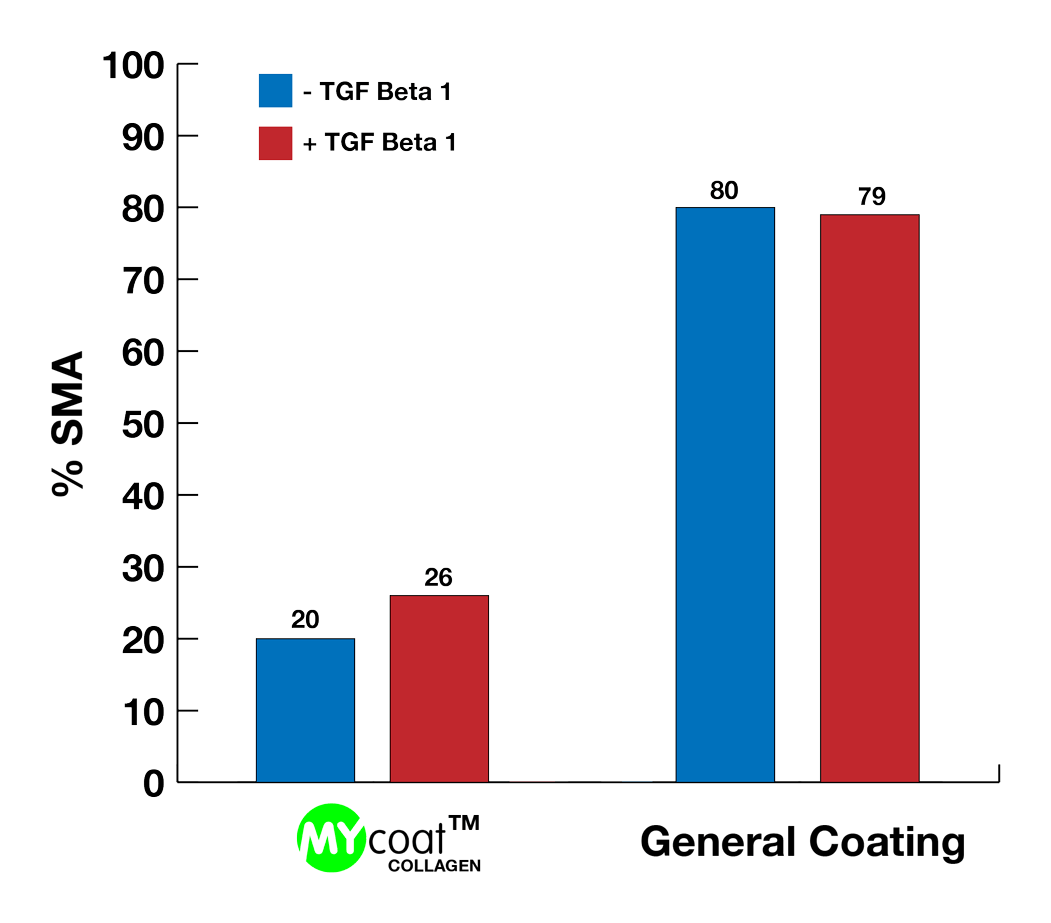
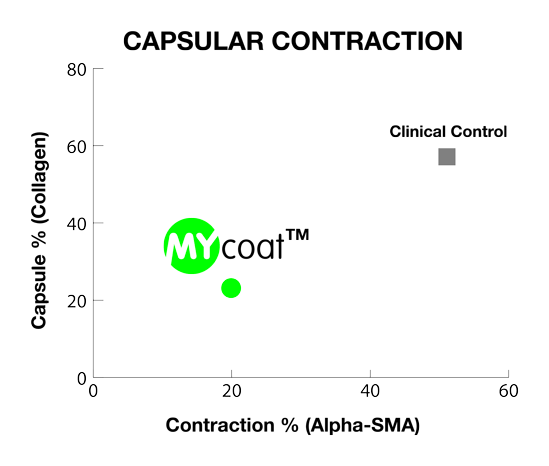
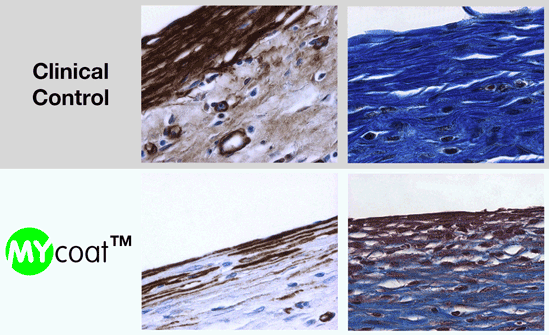
One year post implantation, MYcoat™ silicone implants did not exhibit the formation of a clinically relevant capsule, while standard controls induced the formation of a thick capsule made of scar tissue. The labseed MYcoat™ results show a stable reduction of capsular contraction once the process of acute inflammation around the implant is extinguished. MYcoat™ modulates the first interactions between the implant and host body, guiding the process toward a non-fibrotic type of response.
More details in
Majd, H., et al., Biomaterials 54 (2015), pp. 136-147
Labseed is a spin-off from the École Polytechnique Fédérale de Lausanne (EPFL), in Switzerland, and we became incorporated in 2009.
We developed MYcoat,™ a unique product consisting of a treatment surface which reduces the level of fibrotic reaction and rejection of implantable medical devices.
We are supported by the Swiss Commission for Technology and Innovation (CTI), Discovery Project N° 10447: Smart Coating for Implantable Medical Devices.


Institut de Traumatologie et d’Orthopédie du Léman Suisse

Laboratory of Biomechanical Orthopedics (LBO)
Microsystems Laboratory 4 (LMIS 4)

Laboratory of Tissue Repair and Regeneration

Service de Chirurgie Plastique et de La Main


January 30, 2012
A technology developed by labseed, an EPFL spin-off, could prevent most breast implant rejections.
More than a quarter of all breast implants must be removed within four years, because neighboring tissues develop...

December 2010
Labseed is developing a technology that will make implants more biocompatible.
"It was the ideal solution for bringing our technology to market." As soon as he was convinced of his research's commercial potential...

February 23, 2010
La mise en place d'un implant est perçue comme un corps étranger. Petit à petit, une réaction fribrotique a lieu. Une cicatrice, ou plus exactement une capsule fibrotique, va se former et limiter parfois la fonctionnalité de l'implant. Une nouvelle...
Patent name: Coated medical device and method of coating a medical device to reduce fibrosis and capsule formation.
Patent Number EP2331152. The patent was been granted on 19.05.2017 and published on 21.06.2017
Priority number and date: EP20080163816 (05.09.2008)
Designated contracting states: AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LI, LT, LU, LV, MC, MK, MT, NL, NO, PL, PT, RO, SE, SI, SK, SM, TR.



